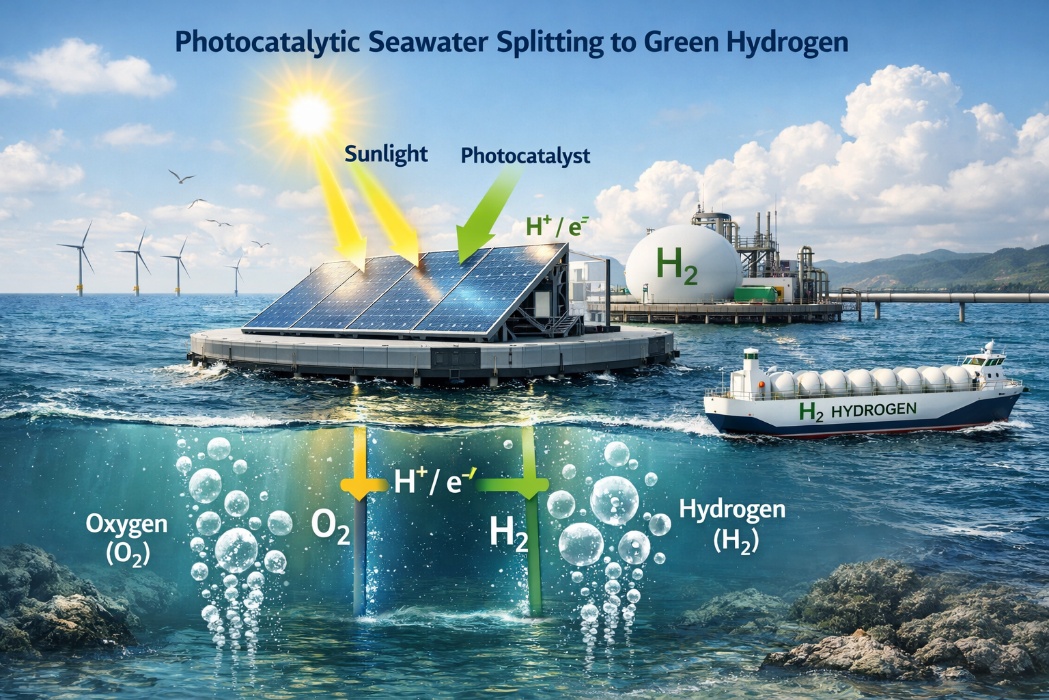
Green hydrogen is widely regarded as a fuel of the future because it produces only water when used and releases no greenhouse gases. Currently, most green hydrogen is produced by splitting freshwater using renewable electricity, which limits large-scale adoption due to growing water scarcity.
To overcome this challenge, a team of European scientists led by Professor Shoubhik Das from the University of Bayreuth, Germany, demonstrated that green hydrogen can be generated using two of the most abundant natural resources on Earth: sunlight and seawater. Their findings were published in the Journal of the American Chemical Society.
The researchers developed a highly dispersed nickel-based photocatalyst with that uses sunlight to split water molecules and release hydrogen. Under laboratory conditions, the system achieved hydrogen production rates of up to 270 micromoles per gram per hour. When tested under direct sunlight, it continued to perform efficiently, producing around 17 micromoles per gram per hour. Importantly, the system was also able to generate hydrogen directly from seawater, reaching production rates of up to 144 micromoles per gram per hour.
In addition to its efficiency, the technology showed excellent durability. The photocatalyst remained stable for over 720 hours of continuous operation, highlighting its potential for long-term, real-world applications.
According to the authors, this breakthrough could enable green hydrogen production along coastlines without competing with drinking or agricultural water resources, supporting global efforts to reduce carbon emissions and achieve net-zero climate goals.