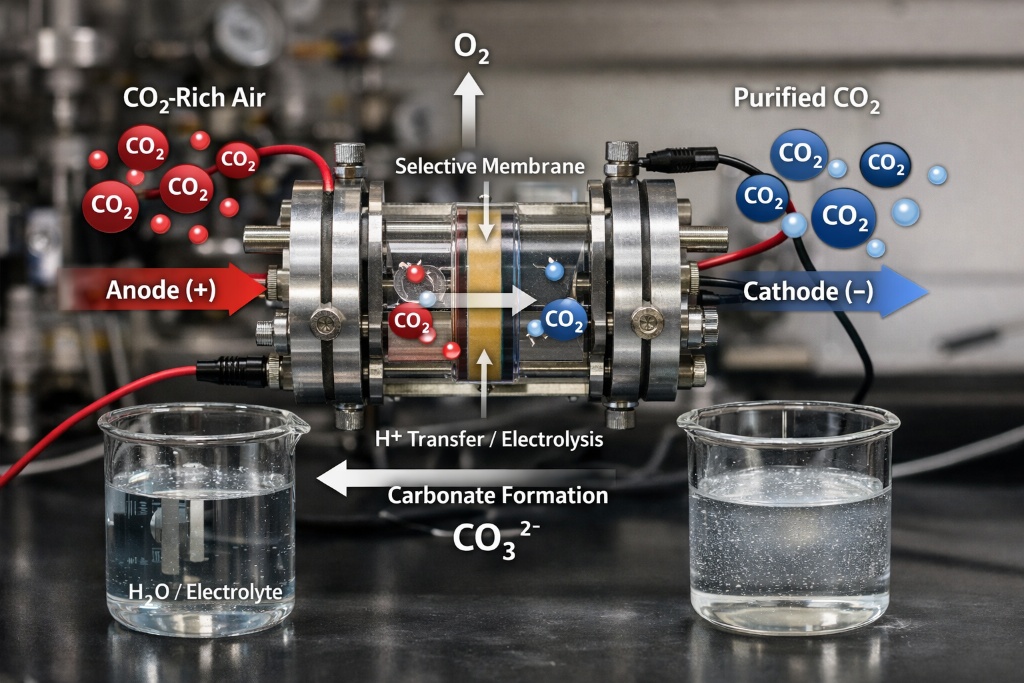
A team of scientists led by Mike Tebyetekerwa and Xiwang Zhang from Australia’s Queensland University has developed a groundbreaking electrochemical carbon capture technology that could dramatically reduce both the energy and time required to remove carbon dioxide from industrial and atmospheric gas streams.
The research, recently published on ChemRxiv, introduces a membrane-integrated supercapacitor system that achieves unprecedented rates of CO₂ capture while consuming minimal energy.
Traditional carbon capture methods are often limited by slow kinetics and high energy demands. By integrating a cation-exchange membrane to separate the electrode compartments, the new system maintains a high concentration of hydroxide ions at the cathode. This creates a localized alkaline interface that rapidly converts CO₂ into (bi)carbonate species through a pH-swing mechanism.
Key Highlights of the Research:
- CO₂ uptake: up to 893 mmol per kilogram of cathode material
- Capture rates: over 1,200 mmol kg⁻¹ h⁻¹
- Energy consumption: as low as 32 kJ per mole of CO₂
- Operational stability: sustained performance over 200+ hours of continuous operation
The study highlights the potential of electrochemical pH-swing strategies to provide scalable, environmentally friendly solutions for mitigating climate change. The authors note that such approaches could complement renewable energy infrastructure by capturing carbon efficiently with minimal additional energy input.